Abstract
BACKGROUND: Platelet alloimmunization and refractoriness is an established complication of platelet (PLT) transfusion, known to be treatment-limiting with potential life-threatening complications for hemato-oncologic patients undergoing chemotherapy (Hess et al.). Previous studies have explored the association of PLT refractoriness with lymphocytotoxic antibody positivity, more frequent platelet transfusion and platelet age (Slichter et al, Blood, 2005). They have also developed cross matching or HLA matching for donor selection as the basis of managing refractory patients. However, the impact of patient characteristics on the clinical outcomes of refractoriness is not fully understood.
Objective: To analyze overall survival (OS) of PLT refractory leukemia patients transfused with HLA-matched platelets in association with the demographic and clinical characteristics of these patients.
METHODS: We analyzed the demographics, clinical characteristics and platelet refractoriness (defined as two consecutive <24 hr post-transfusion corrected platelet count increments (CCI) of < 5 K/uL), ABO matching (fully compatible, minor mismatch or major mismatch), and HLA match grade (A, B, C). The threshold for transfusion was <7 K/uL and only patients with recorded platelet counts pre- and post-transfusion were included. The majority of post-transfusion counts were performed < 24 hrs. Transfusion efficacy was defined as having: i) difference in platelet counts > 5 pre- and post-transfusion; ii) ABO match or compatible; iii) HLA match grade A or B1.
We included two measurements for overall survival (OS): from the date of the first transfusion and also from the last transfusion to the date of last follow-up or date of death. A PLT transfusion record for each measure was obtained using a majority rules algorithm. Group differences in OS were assessed using the log-rank test. For differences in PLT counts, the median value for each patient was used. The association between mean PLT count difference, age, and weight with OS was evaluated using univariate Cox proportional regression models.
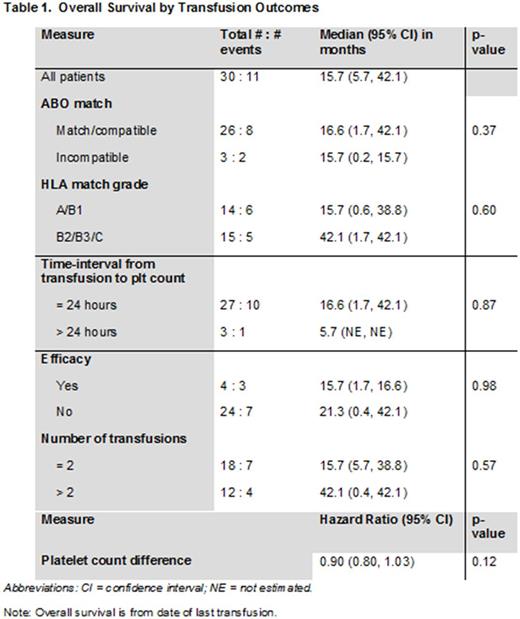
RESULTS: We identified 33 leukemia patients who received a total of 145 HLA-matched platelet transfusions from 2010-2017. A majority [60%] of the patients had a diagnosis of acute myeloid leukemia or myelodysplastic syndrome and received ≤2 HLA-matched platelet transfusions. Complex cytogenetics was identified in 7 of 23 (30%) patients, while the majority (97%) showed broad reactivity against HLA Class I and Class II antigens. Less than half (45%) of the platelets transfused were HLA match grade B and a third of the platelets (35%) were ABO compatible.
The median OS was 16.7 months and the 1-year OS rate was 64%. A significant association between post-transfusion OS with age [p=0.016] and complex cytogenetics [p=0.013] was observed. However, no significant association between OS and gender, weight, ethnicity, and the number of pregnancies was noted. Although the median OS was longer for patients with ABO match/compatible transfusions (vs. incompatible), HLA match grades B2/B3/C platelets (vs. A/B1), and >2 platelets transfusions (vs. ≤ 2), none of these differences were statistically significant (Table 1).
CONCLUSIONS: In summary, our findings reveal a significant association of OS was noted with age and complex cytogenetics in platelet refractory hemato-oncologic patients who received HLA-matched platelet transfusions. This association prompts further investigation into how patient profiles may be used in HLA-alloimmunized patients with platelet refractoriness. However, this may need to be studied in a larger cohort of patients in the future.
DiNardo: Celgene: Honoraria, Research Funding; Agios: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding. Cortes: ImmunoGen: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Teva: Research Funding; Sun Pharma: Research Funding; Pfizer: Consultancy, Research Funding. Kantarjian: Delta-Fly Pharma: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Bristol-Meyers Squibb: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal